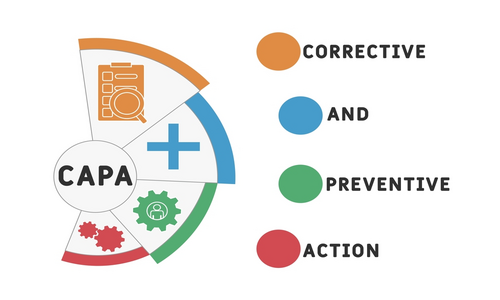
As the name suggests, corrective action denotes the reaction to a situation that has already occurred. On the other hand, preventive action detects the possibility of the occurrence of that situation. The common link between the two is non-conformance when it comes to quality management.
Jump ahead to
What Is Corrective Action?
So, corrective action is a reactive measure to the occurrence of non-conformity in quality management. For instance, failure to meet consumer expectations results in product defects and consumer complaints. To resolve this failure is corrective action. It is undertaken only after the defect has been identified. Corrective action deploys RCA(Root Cause Analysis) to identify the underlying reason and prevent the recurrence of the fault in the future. It assumes the existence of a non-conformity reported through external or internal sources.
Corrective action takes care of severe issues, including safety concerns and recurring problems. It provides several benefits, like increased transparency and a benchmark for future quality management events. The outcome of corrective action is an overall modification of the quality managing approach in catering to consumer demands.
What Is Preventive Action?
Unlike corrective action, preventive action is a proactive measure based on analyzing the trends of non-conformance issues. It begins with a risk assessment of events that are likely to occur on the occasion of improper quality management. Documentation of records or examples of non-conformity is an integral part of preventive action to avoid the occurrence of a similar defect altogether. It eliminates the likeliness of an undesirable future event through the prediction of issues and beforehand planning of remedies.
This is a quality management tool to ensure anticipation, detection, and elimination of problems before their emergence. The efficiency of business operations lies in detecting potential discrepancies. Apart from trend analysis, workers’ participation, reviewing customer feedback, and conducting internal audits are the related practices. These assure control implementation in an organization’s quality management.
Define Non-Conformance In Quality Management
The inability to meet or conform to the specified standards or requirements of a product or service is termed non-conformance. Wrong interpretation of instructions by quality management workers is often the prominent cause of failures in quality management. Flaws in a product and consumer complaints are instances of non-conformance.
A Glance Through Corrective And Preventive Action Processes
Corrective action process
- Detect the prime reason for the occurred non-conformance and then document it.
- Secondly, the entire quality management system must undergo scanning to ensure that no risk exists.
- Analyze the non-conformity first before detecting it.
- Appropriate measures need to be taken to handle the situation, like requesting customers for product returns.
- Sending notifications to consumers regarding the scrapping or downgrading of the product is an effective solution, too.
- A follow-up is essential to ensure the efficiency of the deployed corrective action in preventing future probabilities of recurrences.
Preventive action process
- Gather information from various sources for identifying, assessing, and mitigating potential causes.
- Reports on process operation and consumer feedback are potential sources for gathering this information.
- Identification of steps needed for preventing non-conformance issues.
- Execute the determined preventive steps.
- Keep a record of the actions undertaken and also review them.
The Fine Line Between Preventive And Corrective Actions
Here is a clear view of the subtle difference existing between corrective and preventive actions.
Corrective actions are undertaken only after some issues have emerged regarding quality management. It could be anything, starting from product faults to untimely delivery. The problem has already occurred, and a remedy is needed not just to solve it but keep it at bay.
On the other hand, preventive actions are the results of thoughtful observations of past tendencies and remaining alert. These actions are taken before the occurrence of an issue. Thus, they, too, function in stopping future re-occurrences of the same.
Preventive action emphasizes what can happen, while corrective action deals with the incident that has happened already. The former precedes defect detection while the latter succeeds it. An analysis of a problem’s root marks the beginning of corrective action. On the contrary, preventive action starts with an assessment of the trending risks.
Corrective action calls for record-keeping alongside documentation. Preventive action only requires documentation of the steps initiated. The former is essential for preventing actual non-conformities. Potential nonconformities can only be addressed through preventive action so that they do not ever occur.
Resolving a difficulty and uprooting its cause isn’t much different from detecting and eliminating the cause before the problem occurs. These two actions can’t be called the same either. Hence, a fine line exists between preventive and corrective actions, both of which cannot produce desired results without the other. To master the application of these two concepts, individuals may undergo professional courses like Certified Quality Management Professional Training.